We Are Ironwood
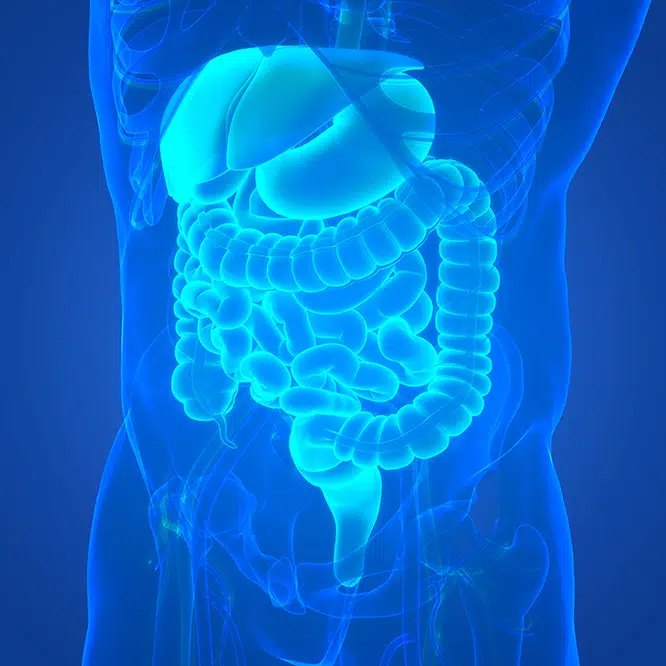
Ironwood Pharmaceuticals is a leading gastrointestinal (GI) healthcare company on a mission to advance the treatment of GI diseases and redefine the standard of care for GI patients.

One in five Americans suffer from GI diseases, resulting in millions of hospital visits and tens of billions in healthcare costs each year.
60 to 70 Million
people affected by all digestive diseases1
48.3 Million
Ambulatory care visits (2010)2–4
21.7 Million
Hospitalizations (2010)5
ADVANCING INNOVATION
Thoughtfully Approaching GI Drug Discovery
Through our singular focus on GI and seasoned GI leadership, we have amassed a great understanding of the unique GI patient journey. These learnings and experiences provide a clear path forward for what we need to do to ensure we continue to reach our goals to deliver value for patients.

-- Christine Brackins, Clinical Sales Specialist, Los Angeles, CA

-- Sheetal Clothe, Senior Manager, Formulation Development

-- Anthony Wajda, Regional Sales Manager

-- Christine Brackins, Clinical Sales Specialist, Los Angeles, CA

-- Sheetal Clothe, Senior Manager, Formulation Development